Greeting: Welcome to this informative article on the Law of Conservation of Energy! In this piece, we will explore this fundamental principle and provide you with two practical examples to help you better understand its application.
The Law of Conservation of Energy is a cornerstone concept in physics that states that energy cannot be created or destroyed. Instead, it can only be converted from one form to another or transferred between objects. This law holds true in all physical processes and is a fundamental principle in understanding how energy behaves in our universe.
📋 Content in this article
To grasp the essence of this law, let’s delve into two practical examples that demonstrate how energy is conserved in different scenarios:
1. Pendulum:
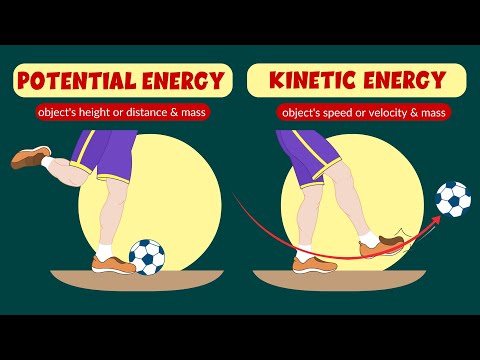
Imagine a simple pendulum hanging from a fixed point. When the pendulum is at its highest point, it possesses potential energy due to its height above the ground. As the pendulum swings downward, this potential energy is converted into kinetic energy, which is the energy of motion. At the lowest point of its swing, all of the potential energy has been converted into kinetic energy. As the pendulum swings back upward, the kinetic energy is gradually converted back into potential energy until it reaches its highest point again. Throughout this entire process, the total amount of energy remains constant, proving the Law of Conservation of Energy.
2. Electric Circuit:
Consider an electric circuit with a light bulb connected to a power source. When the circuit is closed, the power source converts chemical energy into electrical energy and supplies it to the light bulb. The electrical energy is then transformed into light and heat energy in the bulb, illuminating our surroundings. Although some energy may be lost as heat due to resistance in the circuit, the total amount of energy remains unchanged. This example further illustrates how energy is conserved even when it undergoes different conversions.
These examples highlight how energy transformation is at the core of the Law of Conservation of Energy. By recognizing that energy can neither be created nor destroyed, but only transferred or converted, we gain a deeper understanding of the physical processes that govern our world.
In conclusion, the Law of Conservation of Energy is a fundamental principle that underpins many scientific phenomena. It states that energy is always conserved, and it cannot be created or destroyed, only transformed or transferred. The two practical examples we explored—pendulum motion and electric circuits—demonstrate how energy is conserved in different scenarios. Understanding this law is crucial in comprehending how energy behaves and interacts in our universe.
Remember, knowledge of the law empowers us to better understand and appreciate the workings of the world around us!
Understanding the Law of Conservation of Energy: Exploring Real-Life Illustrations
Understanding the Law of Conservation of Energy: 2 Practical Examples
The Law of Conservation of Energy is a fundamental principle in physics that states that energy cannot be created or destroyed, but rather it can only be transferred or transformed from one form to another. This means that the total amount of energy in a closed system remains constant over time. In this article, we will explore two practical examples that illustrate this concept in real-life scenarios.
Example 1: Pendulum Swing
Imagine a simple pendulum consisting of a weight attached to a string. When the weight is lifted and released, it swings back and forth. At the highest points of the swing, the weight has maximum potential energy. As it descends, potential energy is converted into kinetic energy, resulting in an increase in speed. At the lowest point of the swing, all potential energy is converted into kinetic energy. As the weight ascends again, kinetic energy is transformed back into potential energy. Throughout the entire motion, the total amount of energy remains constant.
This example clearly demonstrates the Law of Conservation of Energy as the pendulum continuously converts potential energy to kinetic energy and vice versa. The initial potential energy of the weight is exactly equal to the maximum kinetic energy achieved during the swing.
Example 2: Electric Light Bulb
An electric light bulb provides another practical example of the Law of Conservation of Energy. When an electric current passes through the filament of a light bulb, it encounters resistance which causes the filament to heat up and emit light. The electrical energy is transformed into both heat energy and light energy.
In this case, we can see that the electrical energy supplied to the light bulb is not destroyed but rather converted into different forms of energy. Even though some energy is lost in the form of heat, the total amount of energy input is conserved.
Both of these practical examples highlight how energy is constantly being transformed from one form to another while adhering to the Law of Conservation of Energy. Whether it is the swinging motion of a pendulum or the conversion of electrical energy to light and heat, the total amount of energy within a closed system remains unchanged.
In conclusion, the Law of Conservation of Energy is a fundamental principle that governs the behavior of energy in various systems. Understanding this concept is crucial in many scientific and engineering applications. By exploring real-life illustrations such as pendulum swings and electric light bulbs, we can grasp the practical implications and relevance of this important law.
Understanding the Law of Conservation of Matter: 2 Practical Examples Explained
Understanding the Law of Conservation of Matter: 2 Practical Examples Explained
The Law of Conservation of Matter is a fundamental principle in science that states that matter cannot be created or destroyed in a chemical reaction, but it can only change its form. This law is closely related to the Law of Conservation of Energy, which states that energy cannot be created or destroyed, but only transferred or converted from one form to another.
To better understand the concept of the Law of Conservation of Matter, let’s explore two practical examples:
1. Combustion Reaction:
– When a piece of wood is burned, it undergoes a chemical reaction called combustion. During this process, the wood reacts with oxygen from the air to produce carbon dioxide gas, water vapor, and ash.
– According to the Law of Conservation of Matter, the total mass of the products (carbon dioxide gas, water vapor, and ash) must equal the total mass of the reactant (wood) before the combustion reaction occurred. In other words, no matter is created or destroyed, it is simply transformed into different forms.
– For example, if we weigh the wood before and after the combustion reaction, we will find that the total mass remains the same. This confirms that matter has been conserved.
2. Rusting of Iron:
– Another example that illustrates the Law of Conservation of Matter is the rusting of iron. When iron reacts with oxygen in the presence of water or moisture, it forms iron oxide, commonly known as rust.
– Just like in the combustion reaction example, the Law of Conservation of Matter states that the total mass of the reactants (iron and oxygen) must equal the total mass of the product (iron oxide). The atoms that make up the reactants are rearranged to form new substances, without creating or destroying any matter.
– If we were to measure the mass of the iron before and after it rusts, we would find that the total mass remains the same, further supporting the principle of matter conservation.
In summary, the Law of Conservation of Matter tells us that matter cannot be created or destroyed, but only transformed from one form to another. This principle is evident in various chemical reactions, such as combustion and rusting. By understanding and applying this concept, scientists and engineers can better predict and control chemical reactions and their outcomes.
Remember, if you ever have any legal questions or concerns regarding the application of the Law of Conservation of Matter or any other legal matter, it is always best to consult with a qualified attorney who can provide you with accurate and personalized advice based on your specific situation.
Understanding the Law of Conservation of Energy: Explained with Examples
Understanding the Law of Conservation of Energy: 2 Practical Examples
The concept of the Law of Conservation of Energy is a fundamental principle in the field of physics. It states that energy can neither be created nor destroyed, but it can only be transferred or transformed from one form to another. This principle is based on the understanding that the total amount of energy in a closed system remains constant over time.
To better comprehend this concept, let’s explore two practical examples that demonstrate the Law of Conservation of Energy in action:
1. Pendulum Swing:
Imagine a simple pendulum, consisting of a weight attached to a string. As the weight swings back and forth, it exhibits the Law of Conservation of Energy. At the highest points of its swing, the weight possesses potential energy, which is the energy associated with its position relative to the Earth’s surface.
Key point: At the highest points of its swing, the pendulum has maximum potential energy.
As the weight descends, potential energy is transformed into kinetic energy, which is the energy associated with its motion. At the lowest point of its swing, all potential energy has been converted into kinetic energy.
Key point: At the lowest point of its swing, the pendulum has maximum kinetic energy.
Throughout the pendulum’s motion, the sum of potential and kinetic energy remains constant, showcasing the Law of Conservation of Energy.
2. Roller Coaster Ride:
A roller coaster ride provides another practical example to understand the Law of Conservation of Energy. As the coaster ascends to its highest point, it gains potential energy, which is then converted into kinetic energy as it descends. The coaster’s speed increases due to the conversion of potential energy into kinetic energy.
Key point: At its highest point, the roller coaster has maximum potential energy.
As the coaster approaches the bottom of a hill, it reaches its highest speed due to the conversion of potential energy back into kinetic energy.
Key point: At the bottom of a hill, the roller coaster has maximum kinetic energy.
Throughout the entire ride, the total energy of the roller coaster remains constant, proving the Law of Conservation of Energy.
In conclusion, the Law of Conservation of Energy is a fundamental principle that governs the behavior of energy in a closed system. Two practical examples, the pendulum swing and the roller coaster ride, demonstrate how energy is conserved and transformed from one form to another. By understanding this concept, we can gain a deeper appreciation for the fundamental laws that govern our physical world.
Understanding the Law of Conservation of Energy: 2 Practical Examples
As a seasoned attorney in the United States, I have come to appreciate the importance of staying up-to-date on various topics, including scientific principles like the Law of Conservation of Energy. This fundamental concept plays a significant role in many areas of law, from environmental regulations to contract disputes. In this article, I will explain the Law of Conservation of Energy and provide two practical examples to illustrate its relevance in legal matters.
The Law of Conservation of Energy is a fundamental principle in physics that states that energy cannot be created or destroyed but can only be converted from one form to another or transferred between objects. In simpler terms, the total amount of energy in a closed system remains constant over time. This principle is derived from empirical observations and has been tested and verified through numerous scientific experiments.
In legal contexts, understanding the Law of Conservation of Energy can be crucial for analyzing various cases. It helps us grasp the underlying principles behind certain regulations, liabilities, and responsibilities. By recognizing that energy cannot be created or destroyed, we can better comprehend the impacts of actions and determine who should be held accountable in specific situations.
To further illustrate its importance, let’s consider two practical examples:
While the examples provided above demonstrate the relevance of the Law of Conservation of Energy in legal matters, it is important to verify and contrast the content of this article. As an attorney, it is crucial to conduct thorough research, consult relevant experts, and consider the specific legal context of each case. Laws may vary from jurisdiction to jurisdiction and may be subject to interpretation by courts. Therefore, it is essential to rely on current and accurate legal information when applying scientific principles like the Law of Conservation of Energy in legal arguments.
In conclusion, understanding the Law of Conservation of Energy is vital for attorneys dealing with various legal matters. It provides a foundation for comprehending the consequences of actions and determining legal liabilities. By staying up-to-date on scientific principles like this, attorneys can better serve their clients and contribute to the development of sound legal arguments.
Note: The information provided in this article is for general educational purposes only and should not be considered legal advice. Always consult with a qualified attorney for advice tailored to your specific situation.
